|
Axial Rotation of an Actin Filament Revealed by
Single-Fluorophore Imaging
A
single
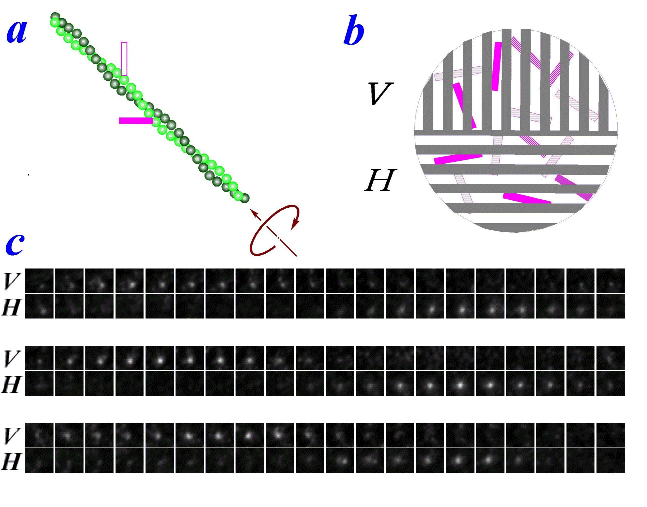
fluorophore of tetramethylrhodamine was attached to an actin filament,
which lay on a surface coated with myosin. The angle between
the long axis of the fluorophore and filament axis happened to be
~45°. When ATP
was added, the filament slid towatd upper left, at ~45°
in the image plane. To detect the orientation of the
fluorophore, we decomposed the fluorescence into vertically (V) and
horizontally (H) polarized components, and imaged the two
simultaneously. As seen in the movie, the fluorescent spot
appeared altenately in the V and H images, showing that the fluorophore
assumed vertical and horizontaly orientations
alternately. The alternation was quite regular, and was
synchronous with the translational movement toward upper
left. The interpretation is that the actin filament rotated
around its axis as it moved past myosin (figure, a). The
filament made one turn per sliding distance of 1 μm. This
rotational pitch is much larger than the helical pitch of actin of 72
nm, suggesting that myosin 'runs' rather than 'walks' on actin.
|
|||||||