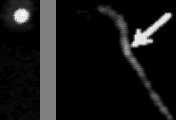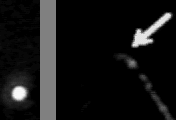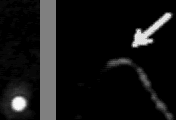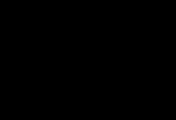
Left: transmitted image
Right: fluorescence image
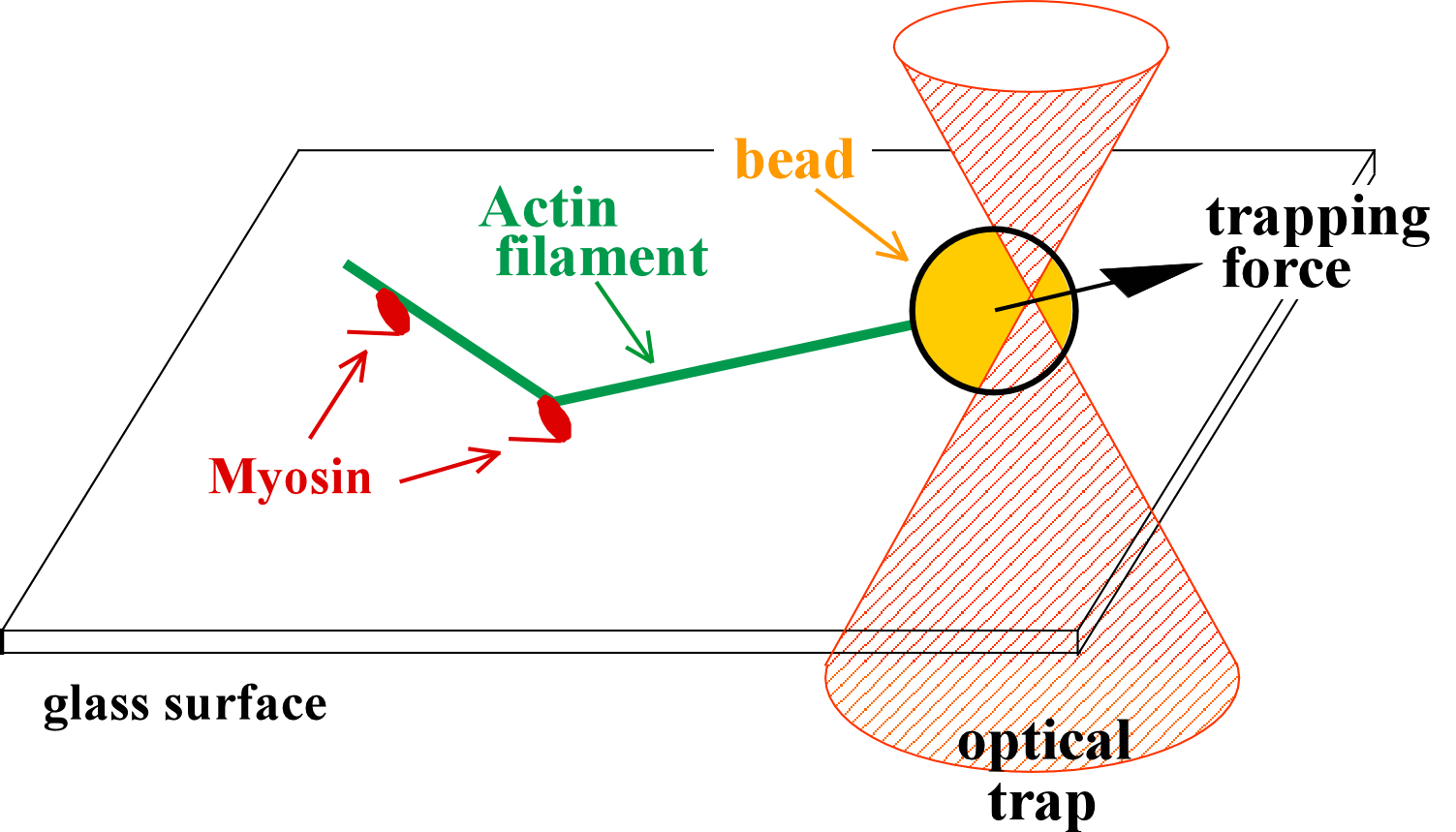
In the absence of ATP, myosin and actin form a tight bond, the 'rigor' bond. To measure the force needed to break a single rigor bond, a plastic bead was attached to one end of a fluorescently stained actin filament and the bead was slowly pulled with optical tweezeres. The movie at the top shows a short actin filament tethered to a single myosin molecule: single-point tether is clearly indicated by the Brownian motion seen in the fluorescence image. When the optical tweezers are turned on, the bead is trapped such that the actin filament is slightly bent. Then, the bead is slowly moved upward. The actin filament becomes straight, and eventually the rigor bond is broken. In the transmitted image on the left, the bead suddenly catches up the trap position when the bond is broken. The unbinding force is estimated from the jump size. Unbinding of a single protein-protein bond is a stochastic event. The external pulling force accelerates the rate of unbinding: higher force results, on the average, in earlier unbinding. The movie at the bottom shows that unbinding and binding can be repeated, indicating that the unbinding is between myosin and actin, not between myosin and the glass surface..